Abstract
Objective
Hematopoietic stem cell transplantation (HSCT) is one of the most efficient treatments for hematologic malignancies. However, relapse remains the main cause of transplant failure. In myeloid neoplasia, the long-term survival for patients who relapsed after HSCT ranges from 10% to 20%. Conventional chemotherapy and donor lymphocyte infusion (DLI) exhibit limited effect and are accompanied by high risks of infection and graft versus host disease (GVHD). New, less toxic, and more efficient treatment options are urgently needed. Venetoclax (VEN), a selective inhibitor of anti-apoptotic protein Bcl-2, combined with hypomethylating agents (HMAs) was well-tolerated and showed an impressive response rate in elderly patients with AML, both as frontline therapy and for relapse. While in post-transplant relapsed patients, the efficacy of the combination of VEN and HMA (VEN-HMA) still needs to be explored.
Methods
Between July 2018 and May 2021, forty-two patients who experienced post-transplant relapse were retrospectively analyzed in this study. All these patients received at least one cycle of VEN-HMA. The salvage therapy consisted of VEN for 28 consecutive days (100 mg of VEN for the first day and 200 mg for the second day, then increased to the final dose of 400 mg daily or equivalent with azole co-administration). Thirty-nine patients received azacytidine (100mg/m2, d1-7) while the other 3 patients were given decitabine (10mg/m2, d1-5) in combination with VEN.
Disease types included acute myeloid leukemia (AML, n=32), myelodysplastic syndrome (MDS, n=7), chronic myelomonocytic leukemia (CMML, n=2) and chronic myeloid leukemia (CML, n=1). 28 (66.7%) patients achieved complete remission before transplantation and 14 (33.3%) patients were transplanted in a non-remission status. Haploidentical-related donor (HID, n=31) was the most common donor type, followed by matched sibling donor (MSD, n=6) and unrelated donor (URD, n=5). Each patient was analyzed for chromosomal and genetic abnormalities either at diagnosis and at post-transplant relapse. Complex/monosomal karyotype was seen in 10 (23.8%) patients. 9 (21.4%) patients had TP53 mutation or deletion and 7 (17.9%) had IDH1/2 mutation. The median time from transplantation to relapse was 329 (range, 30-1584) days, 27 (64.3%) patients had an early relapse (within 1-year post-HSCT). 16 (38.1%) patients developed acute GVHD and 15 (35.7%) developed chronic GVHD before relapse.
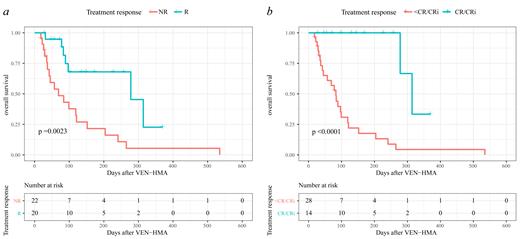
24 (57.1%) patients received VEN-HMA as first-line salvage therapy, while the other 18 (42.9%) patients received this combination therapy after failure with chemotherapy or DLI. 22 (52.4%) patients had a higher tumor burden (bone marrow blasts >20%) at the initiation of VEN-HMA. In total, 20 (47.6%) patients responded to the VEN-HMA therapy with a reduction in bone marrow blasts. 14 (33.3%) patients fulfilled the criteria of CR/CRi and all of them achieved the best response in the first cycle of VEN-HMA. However, 4 (9.5%) patients even experienced disease progression after VEN-HMA. After a median follow-up time of 84 (18-535) days, patients who achieved CR/CRi experienced a significantly higher overall survival (OS) than those who did not (85.7% vs 14.3%, p <0.0001). In univariate and multivariate analysis, we found that early relapse (HR=4.000, p=0.046) was the only independent risk factor that influenced the CR/CRi rate.
Conclusion
VEN-HMA is safe and efficacious for patients with myeloid malignancies relapsing after transplantation. Patients relapsing within 1-year post-transplantation may be more likely to benefit from this combination therapy.
No relevant conflicts of interest to declare.